Influenza & COVID-19 Ag Combo Rapid Test Cassette (Swab)
Influenza & COVID-19 Ag Combo Rapid Test Cassette (Swab)

INTENDED USE
The Influenza & COVID-19 Ag Combo Rapid Test Cassette (Swab) is an in vitro immunochromatographic assay for the qualitative and differential detection of nucleocapsid protein antigen from influenza A (including the subtype H1N1), influenza B and/or SARS-CoV-2 in nasopharyngeal (NP) swab specimens. It is intended to aid in the rapid diagnosis of influenza A, influenza B and/or SARS-CoV-2 infections. This test provides only a preliminary test result. Therefore, any reactive specimen with the Influenza & COVID-19 Ag Combo Rapid Test Cassette (Swab) must be confirmed with alternative testing method(s) and clinical findings.
SUMMARY AND EXPLANATION
Influenza is an acute and highly contagious viral infection of the respiratory tract. The causative agents of the disease are immunologically diverse, single-strand RNA virus known as influenza viruses. There are three types of influenza viruses: A, B and C. Type A viruses are the most prevalent and are associated with most serious epidemics, while Type B infection is generally milder. Type C virus have never been associated with a large epidemic of human disease. Both type A and B viruses can circulate simultaneously, but usually one type is dominant during a given season and particular epidemic area. The disease is easily transmitted through coughing and sneezing of aerosolized droplets containing live virus. Influenza outbreaks normally occur each year during fall and winter seasons.
infectious disease. Entire human population is susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue, dry cough, and loss of taste and smell. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases. This test is for detection of SARS-CoV-2 nucleocapsid protein antigen. Antigen is generally detectable in upper respiratory specimens during the acute phase of infection. Rapid diagnosis of SARS-CoV-2 infection will help healthcare professionals to treat patients and control the disease more efficiently and effectively.
PERFORMANCE CHARACTERISTICS
For FluA&B Antigen Rapid Test:- 1. Analytical Sensitivity
The minimum detection limit is 1.5 x 104 TCID50 /test for the Influenza A virus antigen and is 1.5 x 105 TCID50 /test for the Influenza B virus antigen.
- 2. Analytical Reactivity
The influenza A strain listed tested positive in the Influenza A &B Ag Rapid Test. Although the specific influenza strains causing infection in human can very, all contain the conserved nucleoproteins targeted by Influenza A&B Ag Rapid Test.
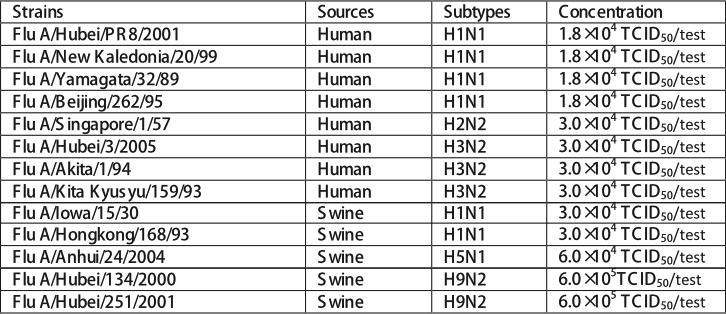
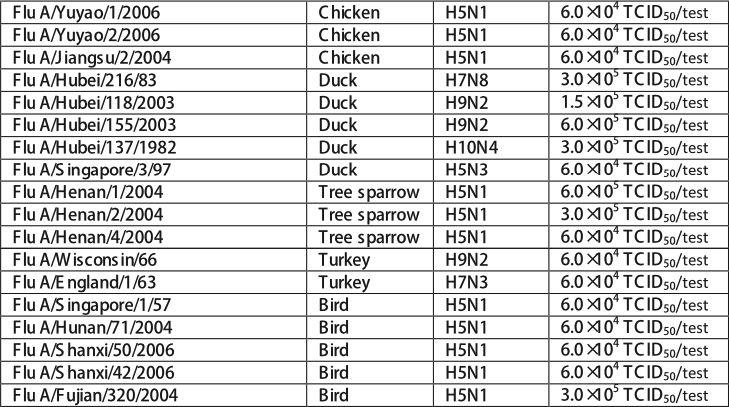
Influenza A&B Ag Rapid Test can detect all nine influenza B strains.
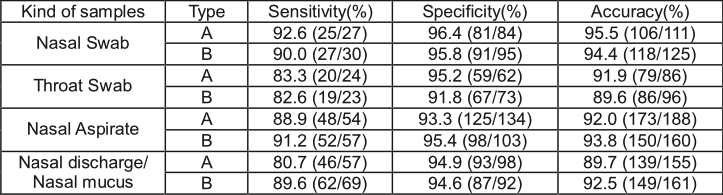
- 3. Clinical Study Data Summary
The Influenza A&B Ag Rapid Test performance vs. Cell Culture
- 4. Analytical Specificity And Cross-reactivity
The Influenza A&B Ag Rapid Test was evaluated with a total of 30 bacterial and viral isolates. Bacterial isolates were evaluated at a concentration between 107 and 109 org/mL. Viral isolates were evaluated at a concentration of at least 104-108 TCID50/mL. Adenovirus 18 and Parainfluenza virus 3 were tested at 102 TCID50/mL. None of the organisms or viruses listed below gave a positive result in the Influenza A&B Ag Rapid Test.
Bacterial Panel:
Acinetobacter calcoaceticusBacteroides fragilisNeisseria gonorrhoeaeNeisseria meningitidisPseudomonas aeruginosaStaphylococcus aureusStreptococcus pneumoniaeStreptococcus sanguisProteus vulgarisStreptococcus sp. Gp. BStreptococcus sp. Gp. CStreptococcus sp. Gp. GMycobacterium tuberculosisMycoplasma oraleViral Panel:
Human Adenovirus BHuman Rhinovirus 2Human Adenovirus CHuman Rhinovirus 14Adenovirus type 10Human Rhinovirus 16Adenovirus type 18MeaslesHuman Coronavirus 0C43MumpsHuman Coxsackievirus A9Sendai virusCoxsackievirus B5Parainfluenza virus 2Human herpesvirus2Parainfl uenza virus 3
- 5. Interfering Substances
Whole blood, and several over-the-counter (OTC) products and common chemicals were evaluated and did not interfere with the Influenza A&B Ag Rapid Test at the levels tested: whole blood (2%); three OTC mouthwashes (25%); three OTC throat drops (25%); three OTC nasal sprays (10%); 4-Acetamidophenol (10mg/mL); Acetylsalicylic Acid (20mg/mL); Chlorpheniramine (5mg/mL); Dextromethorphan (10mg/mL); Diphenhydramine (5 mg/mL); Ephedrine (20mg/mL); Guaiacol glyceryl ether (20mg/mL); Oxymetazoline (10mg/mL); Phenylephrine (100 mg/mL); and Phenylpropanolamine (20mg/mL).
For COVID-19 Antigen Rapid Test:
- 1.Clinical Sensitivity, Specificity and Accuracy
The COVID-19 Antigen Rapid Test Cassette (Swab) has been evaluated with specimens obtained from patients. A commercialized molecular assay was used as the reference method. The results show that the COVID-19 Antigen Rapid Test Cassette (Swab) has a high overall relative accuracy.
Table 1: The COVID-19 Antigen Rapid Test vs PCR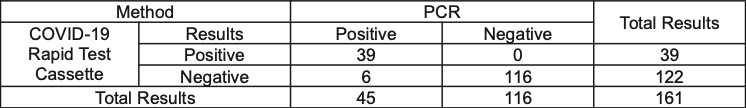
- Relative Sensitivity: 86.7% (95%CI*: 73.2%-95.0%)
- Relative Specificity: 100% (95%CI*: 96.9%-100%)
- Accuracy: 96.3% (95%CI*: 92.1%-98.6%)
INDEX OF SYMBOLS
- 1.Clinical Sensitivity, Specificity and Accuracy