SARS-CoV-2 and Influenza A/B RT-qPCR Detection Kit
SARS-CoV-2 and Influenza A/B RT-qPCR Detection Kit

Concomitant detection of SARS-CoV-2 and Flu-A/B
In Vitro Diagnostic Medical Device
Intended Use: Presumptive qualitative detection of RNA from SARS-CoV-2 (2019-nCoV) and infuenza A/B in specimens from individuals suspected of COVID-19 by their healthcare provider.
THE CHALLENGES
- Differential Diagnosis: Since COVID-19 symptoms can be easily confused for other respiratory infections, diagnostics are urgently needed to distinguish SARS-CoV-2 from influenza A/B virus.
- High Throughput: With the rapid spread of COVID-19, conventional manual nucleic acid extraction can hardly support rapid high-throughput testing.
OUR SOLUTION
- Multi-target design to minimize misdetection.
- Inclusion of Flu A/B as targets to facilitate differentiation between COVID-19 and Flu A/B.
- No cross-reaction with SARS-CoV or MERS-CoV.
The Automated Nucleic Acid Extraction System & the SARS-CoV-2 and Influenza A/B RT-qPCR Detection Kit increase daily throughput by 5-25 times.
THE IMPACT
- Multi-target: With the aim of minimizing false calling, primers and probes are designed to simultaneously target sequences specific of SARS-CoV-2 (ORF1ab and N gene), Flu A (M gene), and FluB ( NP gene) in one signle assay.
- LoD= 5 copies/reaction: SARS-CoV-2 could be present at very low viral titers in some specimens , especially those collected from patients with early infection. A virus detection assay with high sensitivity can significantly reduce false negative.
- Real-world clinical evaluation: RNA samples extracted from 282 clinical specimens were sent for SARS-CoV-2 testing using both next generation sequencing (NGS) and the SARS-CoV-2 and Influenza A/B RT-qPCR Detection Kit. The performance of the kit was evaluated against NGS.
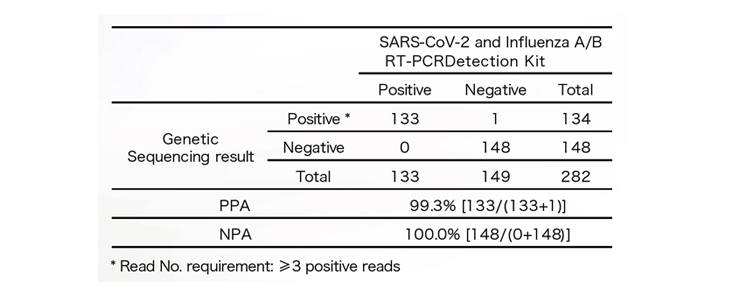
- Inclusivity and specificity: The test cove rs 100% of the known SARS-CoV-2 sequences based on NCBI & GISAID database as of February 20, 2020. No cross-reaction was observed with other pathogens commonly found in respiratory specimens.
- Multiple controls: A positive control and a negative control are included in each run to ensure that the assay is working properly and to identify potential reagent contamination, respectively. An internal control is included in each reaction to monitor reverse transcription and PCR amplification.
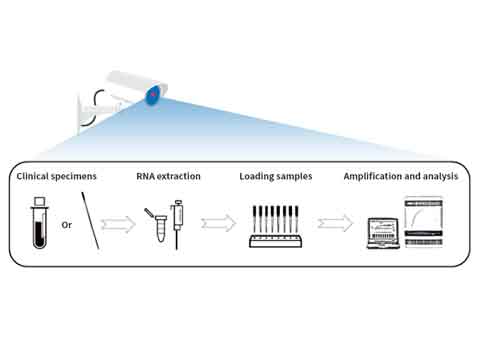
WORKFLOW
The SARS−CoV−2 and Influenza AƒB RT−qPCR Detection Kit is recommended to be used in conjunction with the Automated Nucleic Acid Extraction System (or QIAamp DSP Viral RNA Mini Kit) and the ABI 7500 Real−Time PCR Instrument. Otherwise, a real−time PCR instrument capable of detecting FAM, ROX, VIC, CY5 and TAMRA is required.
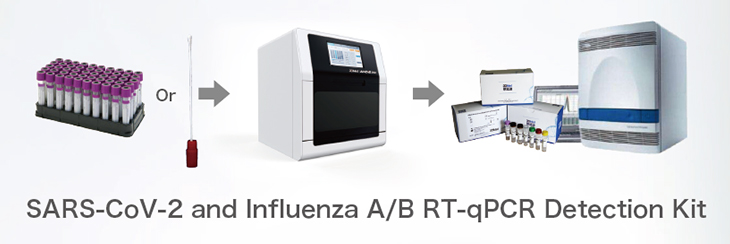
Technology
Real−time Quantitative Polymerase Chain Reaction (qPCR)
Components
- RT−qPCR Reaction Mix
- Enzyme Mix
- SARS−CoV−2 Assay
- Influenza AƒB Assay
- Positive Control, Negative Control and Internal Control
Required Materials (not included)
- RNA Extraction & Purification
- Automated Nucleic Acid Extraction System (Instrument + Reagents)
- Or QIAamp DSP Viral RNA Mini Kit
- ABI 7500 Real−Time PCR System (or equivalent)
- Other laboratory supplies for molecular testing
- Personal Protective Equipment
3 ways to minimize false detection.
High sensitivity: capable of detecting as low as 5 copies/reaction
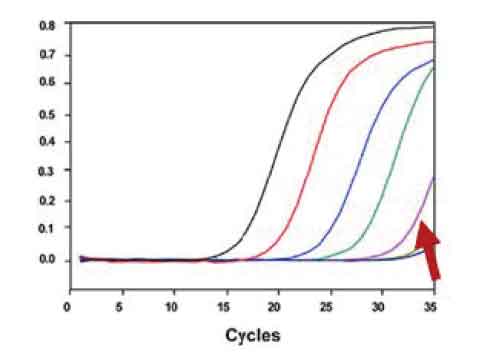
Clinical observations showed low viral titer in the specimens of some patients, especially those at early stage of the infection. A virus detection assay with high sensitivity can significantlly lower the false negative rate.
Multi-target: minimize false calling
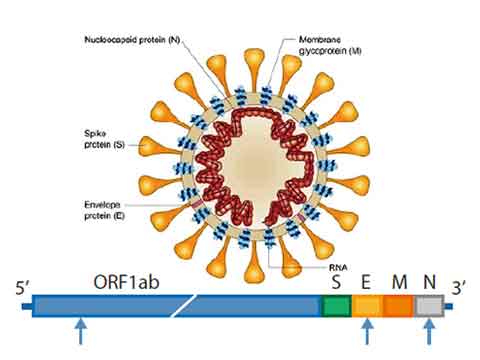
The assay was designed to detect signals from the ORF1ab, N and E genes of 2019-nCoV. The multi-target design can minimize false detection.
Controls: ensure the validity of the test
Positive control: verify the functionality of the assay.
Negative control: indicate the contamination of the reagents.
Internal control: ensure the integrity of 2019-nCoV specific amplification.
Distinguish 2019-nCoV from flu viruses
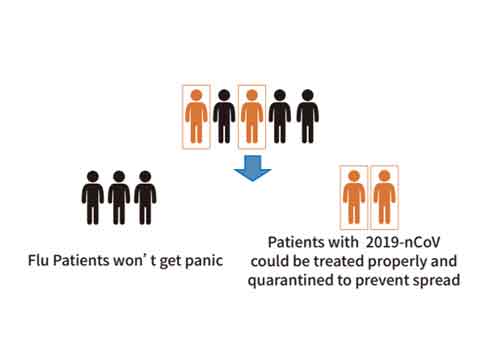
Most patients with 2019-nCoV infection have flu-like symptoms, such as fever, fatigue and cough. Distinguishing the infection of 2019-nCoV from Flu A/B infection is critical for providing better treatment to the patients.
Performance validated by over 1,000 clinical samples
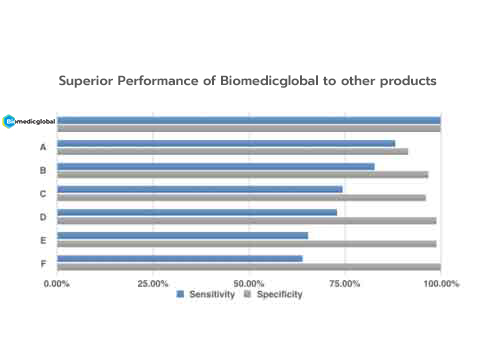
2019-nCoV RT-qPCR Detection Kit exceled in comparison with assays from other companies by testing the real-world clinical samples.