Coronavirus Ag Rapid Test kit
Coronavirus Ag Rapid Test kit

COVID-19 OVERVIEW
The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
Antigen is generally detectable in upper respiratory specimens during the acute phase of infection. Rapid diagnosis of SARS-CoV-2 infection will help healthcare professionals to treat patients and control the disease more efficiently and effectively.
Product Information
The Coronavirus Ag Rapid Test Cassette (Swab) is an in vitro immunochromatographic assay for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2 in nasopharyngeal (NP) swab specimens directly or after the swabs have been added to viral transport media from individuals who are suspected of COVID-19 by their healthcare provider. It is intended to aid in the rapid diagnosis of SARS-CoV-2 infections. The Coronavirus Ag Rapid Test Cassette (Swab) does not differentiate between SARS-CoV and SARS-CoV-2.
Benefits
- Rapid testing for SARS-CoV-2 antigen within 15 minutes
- Facilitates patient treatment decisions quickly
- Simple, time-saving procedure
- All necessary reagents provided & no equipment needed
- High sensitivity and specificity
Specification
Contents
- 20 Test cassettes
- 20 Sterile swabs
- 20 Extraction tubes and dropper tips
- 1 Workstation
- 1 Buffer
- 1 Package insert
Performance Characteristics
The Coronavirus Ag Rapid Test Cassette (Swab) has been evaluated with specimens obtained from patients. A commercialized molecular assay was used as the reference method. The results show that the Coronavirus Ag Rapid Test Cassette (Swab) has a high overall relative accuracy.
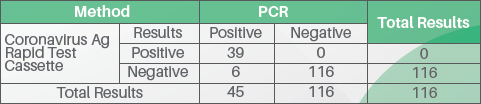
- Relative Sensitivity: 86.7%
- Relative Specificity: 100%
- Accuracy: 96.3%
Test Procedure & Interpretation
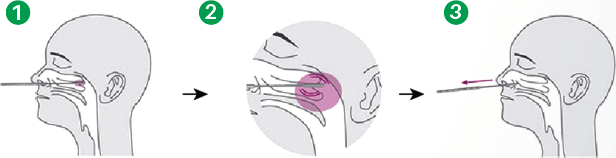
Specimen collection (Use the nasopharyngeal swab supplied in the kit.)
- Carefully insert the swab into the nostril of the patient, reaching the surface of posterior nasopharynx. that presents the most secretion under visual inspection.
- Swab over the surface of the posterior nasopharynx. Rotate the swab several times.
- Withdraw the swab from the nasal cavity.
Sample preparation
- 1.Insert the test extraction tube into the workstation in this product. Make sure that the tube is standing firm and reaches the bottom of the workstation.
- 2.Swab over the surface of the posterior nasopharynx. Rotate the swab several times.
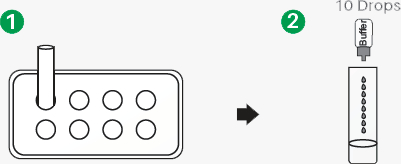
- 3.Insert the swab into the extraction tube which contains 0.3 mL of the extraction buffer. Roll the swab at least 6 times while pressing the head against the bottom and side of the extraction tube.
- 4.Leave the swab in the extraction tube for 1 minute.
- 5.Squeeze the tube several times with fingers from outside of the tube to immerse the swab. Remove the swab. The extracted solution will be used as test sample.
- 6.Fit the dropper tip with filter on top of the extraction tube tightly.
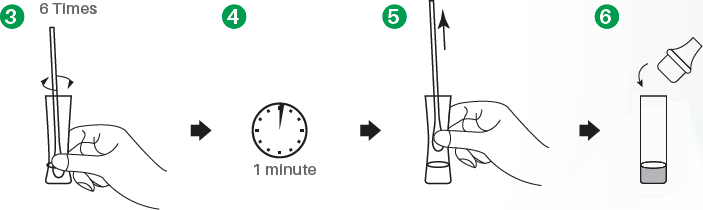
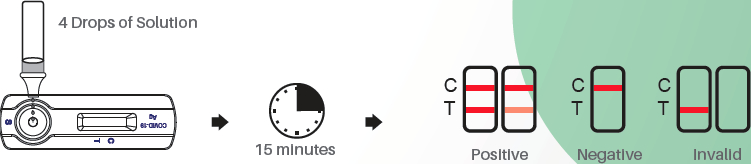
Test procedure & interpretation of results
Ordering Information
